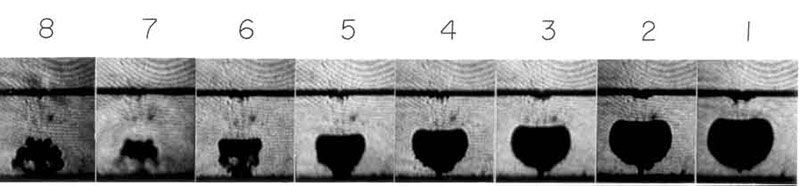
The process of formation of “micro cavities”, otherwise known as cavitation, occurs mainly where the velocity of the liquid is maximum: the liquid mass loses continuity, creating a gaseous “foam”, particularly rich in oxygen, due to steam and air that are released.
This gaseous “foam”, in the event of uncontrolled cavitation, can be extremely erosive and corrosive with metals due the developping of hydrolysis, oxidation, polymerization and depolymerization.
The very rapid collapse of the “micro cavities” generates micro-jets at very high pressure and high concentrations of energy in very short times and spaces which, if not controlled as described above, can cause even considerable damage to the pipes and / or to moving parts of the machines that trigger this phenomenon.
- By way of example, in relation to a pipe, the phenomenon of cavitation can develop more in the sections where the piezometric line falls below the axis of the pipe itself, thus forming a more or less pronounced depression.
- By way of example, in relation to a hydraulic machine (centrifugal, axial pumps, turbines, etc.), the phenomenon of cavitation can develop more in the external points of the impeller where the higher the speed and the lower the pressure.
Cavitation generates friction and turbulence in the liquid causing, if not properly controlled, a significant loss of efficiency, emission of noise, vibrations and damage to components. The decrease in efficiency and power can be greater than 3% compared to similar conditions in the absence of cavitation.
Although the process is similar to the better known one of boiling, the main difference between cavitation and boiling lies in the fact that in boiling, due to the increase in temperature, the vapor pressure rises until it exceeds the pressure of the liquid, thus creating a mechanically stable bubble as it is full of vapor at the same pressure as the surrounding liquid.
In cavitation, on the other hand, the pressure of the liquid suddenly drops, while the temperature and vapor pressure remain constant.
For this reason, the cavitation “bubble” only resists until it leaves the low hydrostatic pressure zone: as soon as it returns to an area of the fluid at rest, the vapor pressure is not sufficient to counteract the hydrostatic pressure and the cavitation bubble implodes, releasing a large amount of energy and the associated shock wave sequence.
The vapor pressure of a liquid is the partial pressure of the vapor when the equilibrium between liquid and vapor is established, it depends on the temperature and increases with it (for water it is 4.6 mmHg at 0° C and 760 mmHg at 100° C).
Once this pressure has been reached, the liquid and the vapor are defined as be saturated (as many molecules pass from the liquid phase to the vapor phase as there are those that carry out the reverse process).
In addition, the cavitation heating is released uniformly over the entire volume of the liquid while a conventional heating takes place by transfer and therefore from a point towards the most extreme face.
This allows you to eliminate hot or cold spots, burns and, if necessary, have precise temperature control.