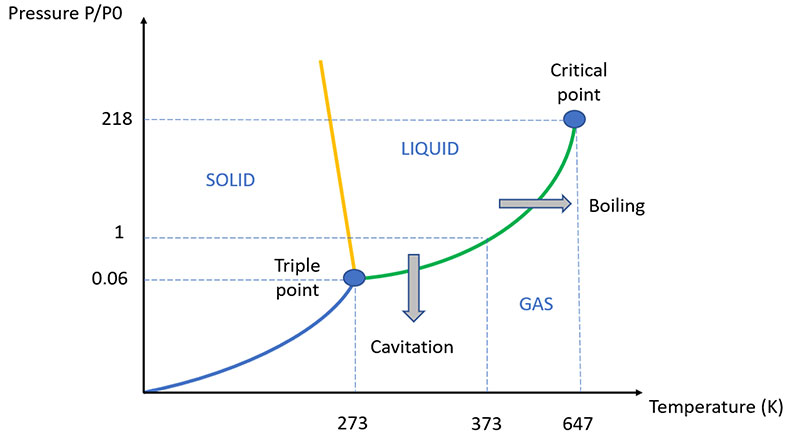
Although the process is similar to the best known one of boiling, the main difference between cavitation and boiling is that in boiling, due to the increase in temperature, the pressure of the vapor rises until it exceeds the pressure of the liquid, thus creating a mechanically stable bubble as it is full of steam at the same pressure as the surrounding liquid.
In cavitation, on the other hand, it is the pressure of the liquid that suddenly drops, remaining constant temperature and vapor pressure. For this reason, the cavitation "bubble" resists only until it leaves the low hydrostatic pressure zone: as soon as it returns to a quiet area of the fluid, the vapor pressure is not sufficient to counteract the hydrostatic pressure and the cavitation bubble implodes releasing a large amount of energy and the related shock wave sequence.
The vapor pressure of a liquid is the partial pressure of the vapor when the balance between liquid and vapor is established, it depends on the temperature and grows with it (for water it is 4.6 mmHg at 0 ° C and 760 mmHg at 100 ° C).
Once this pressure is reached, the liquid and the vapor are said to be saturated (there are as many molecules that pass from the liquid phase to the vapor phase as there are those that perform the reverse process).
In addition, the cavitation heating is released uniformly over the entire volume of the liquid while a conventional heating takes place by "transfer" and, therefore, from a point to the most extreme part. This allows to eliminate hot or cold spots, burns and, if necessary, to have a precise temperature control.